Structure and Cellular Dynamics of Deinococcus radiodurans Single-stranded DNA (ssDNA)-binding Protein (SSB)-DNA Complexes.
George, N.P., Ngo, K.V., Chitteni-Pattu, S., Norais, C.A., Battista, J.R., Cox, M.M., Keck, J.L.(2012) J Biol Chem 287: 22123-22132
- PubMed: 22570477
- DOI: https://doi.org/10.1074/jbc.M112.367573
- Primary Citation of Related Structures:
3UDG - PubMed Abstract:
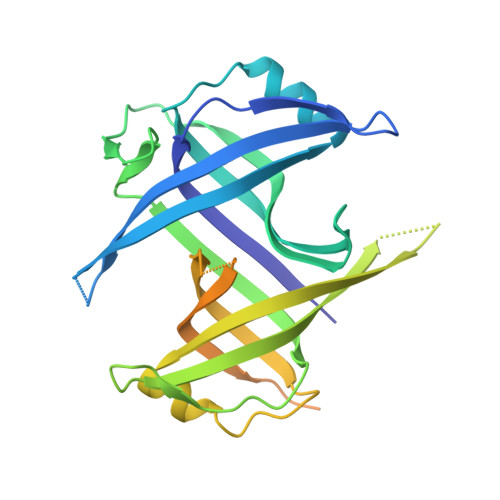
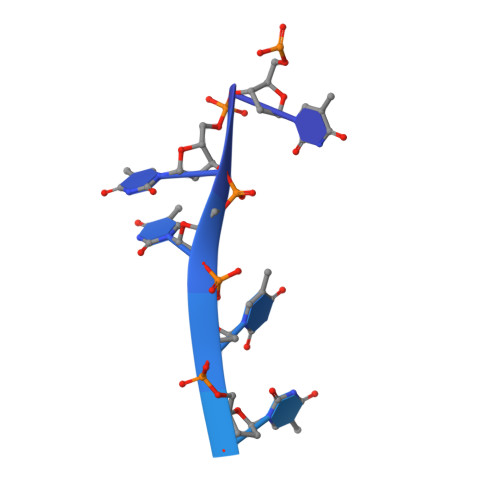
The single-stranded DNA (ssDNA)-binding protein from the radiation-resistant bacterium Deinococcus radiodurans (DrSSB) functions as a homodimer in which each monomer contains two oligonucleotide-binding (OB) domains. This arrangement is exceedingly rare among bacterial SSBs, which typically form homotetramers of single-OB domain subunits. To better understand how this unusual structure influences the DNA binding and biological functions of DrSSB in D. radiodurans radiation resistance, we have examined the structure of DrSSB in complex with ssDNA and the DNA damage-dependent cellular dynamics of DrSSB. The x-ray crystal structure of the DrSSB-ssDNA complex shows that ssDNA binds to surfaces of DrSSB that are analogous to those mapped in homotetrameric SSBs, although there are distinct contacts in DrSSB that mediate species-specific ssDNA binding. Observations by electron microscopy reveal two salt-dependent ssDNA-binding modes for DrSSB that strongly resemble those of the homotetrameric Escherichia coli SSB, further supporting a shared overall DNA binding mechanism between the two classes of bacterial SSBs. In vivo, DrSSB levels are heavily induced following exposure to ionizing radiation. This accumulation is accompanied by dramatic time-dependent DrSSB cellular dynamics in which a single nucleoid-centric focus of DrSSB is observed within 1 h of irradiation but is dispersed by 3 h after irradiation. These kinetics parallel those of D. radiodurans postirradiation genome reconstitution, suggesting that DrSSB dynamics could play important organizational roles in DNA repair.
Organizational Affiliation:
Department of Biomolecular Chemistry, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin 53706, USA.